São Paulo-based firm creates “artificial lung” to treat COVID-19
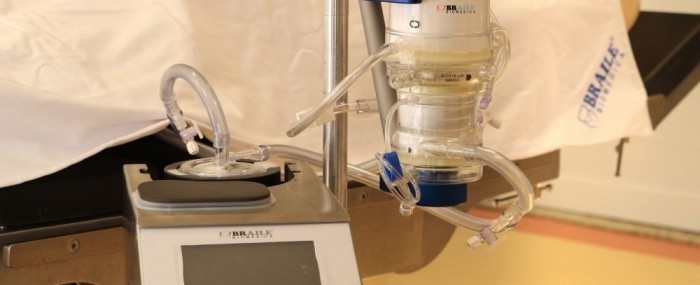
16 de fevereiro de 2021By Yuri Vasconcelos* | FAPESP Innovative R&D – Braile Biomédica, one of the first Brazilian companies to develop medical equipment for cardiology, has just brought to market a highly complex medical device that will help treat critical COVID-19 patients. Known as Solis, it is an extracorporeal membrane oxygenation (ECMO) system in which blood is pumped through a heart-lung machine that removes carbon dioxide and returned full of oxygen to keep the patient’s cardiopulmonary system going when a mechanical ventilator can no longer provide adequate gas exchange or perfusion to sustain life.
“It’s a sort of artificial lung that can be used in adults and children,” says Rafael Braile, mechanical engineer, chief operating officer and head of R&D at the firm, which is headquartered in São José do Rio Preto, state of São Paulo. “The blood exits and returns to the patient’s body via catheters, and is oxygenated in the machine with the aid of a polymeric membrane. The system provides invasive life support to facilitate the patient’s survival until the lungs recover.”
ECMO machines like Solis are indicated in cases involving heart transplants and for patients who have had a heart attack or cardiac arrest. They can also be used to assist severe COVID-19 patients with severe acute respiratory failure.
Braile developed Solis in less than a year with technical support from EMBRAPII’s Eldorado Research Institute in Campinas, São Paulo. EMBRAPII is the Brazilian Company of Research and Industrial Innovation, a federal agency. It invested BRL 2.3 million in the project. Braile invested a matching amount. The São Paulo State Development Agency (Desenvolve SP) provided BRL 2.5 million and the National Development Bank (BNDES) supplied BRL 3 million.
Founded by cardiovascular surgeon Domingo Marcolino Braile (1938-2020) in 1977, the firm was awarded funding by FAPESP under its Innovative Research in Small Business Program (PIPE) to develop a peripheral stent (a scaffold inserted into peripheral blood vessels to treat blockage), and already had the know-how to manufacture similar equipment. “Back in the 1990s we mastered the technology to manufacture cardiac surgery membrane oxygenators that do the work of the lungs for a shorter period, typically not exceeding six to eight hours. The Solis design concept is similar, except it has to keep running for 30 days or more, for as long as the patient’s cardiorespiratory system is recovering in intensive care,” Braile says, adding that Solis is the first ECMO machine developed locally in the southern hemisphere.
Braile uses cutting-edge technology, according to Luiz Fernando Canêo, a pediatric heart surgeon at the Heart Institute (INCOR) of Hospital das Clínicas (HC), the hospital complex run by the University of São Paulo’s Medical School (FM-USP). “Their machine has to compete with what’s already on the market. All ECMO machines in Brazil are currently imported. Solis is a significant achievement,” says Canêo, who chaired the Latin America section of the Extracorporeal Life Support Organization (ELSO) between 2015 and 2018.
The dozen or so companies supplying the global market for ECMO machines include Getinge (Sweden), Medtronic (Ireland), LivaNova (UK), Nipro (Japan) and Terumo (USA). According to Canêo, the Brazilian device is theoretically as good as theirs. “Now we need clinical experience to prove it performs well,” he says. The price of Solis has not yet been disclosed, but similar machines cost USD 35,000-USD 50,000 on the world market.
The firm has sold one unit to a medical equipment distributor based in the state of Pernambuco in Northeast Brazil. It will be installed at a hospital in the state of Ceará. “We’ve received inquiries from several Brazilian states and from abroad. We’re at an advanced stage of talks with centers in Germany and Ukraine. Institutions in Colombia and Venezuela have also expressed interest in Solis,” Braile says.
Disposable parts
An ECMO machine has two main components. One controls parameters such as blood flow, pressure and temperature. The other is a cluster of disposable parts, such as cannulas (catheters) to drain and return blood, tubes to transport the blood, a centrifuge pump, and a membrane oxygenator for gas exchange. Disposable items are swapped out with each new patient.
“Our system is the only one that comprises all the items required for an ECMO machine. We make all the parts,” Braile says. “Our main competitors don’t develop everything. They acquire some components from suppliers.”
Another innovation in Solis, he adds, is the inner lining of all parts with a recombinant albumin biofilm that prevents thrombosis and blood clots.
ECMO therapy was widely used during the 2009-10 H1N1 flu pandemic, he notes. The results were positive then, just as they are now in Europe and the US, where ECMO machines are being used to help treat COVID-19 patients. “The mortality curve has been lower in these countries. We expect our system to save lives during the ongoing coronavirus pandemic,” he says.
Few Brazilian hospitals are equipped to perform ECMO because it is a complex procedure that requires highly trained personnel and also owing to its high cost, “which is not always covered by medical insurance policies” Canêo remarks. “Only 28 medical centers are members of ELSO and report their activities to the organization. Membership is a hallmark of quality.” Non-members of ELSO also perform ECMO therapy in Brazil.
According to Canêo, a supply of indigenous equipment can help expand the use of ECMO therapy in Brazil. “There’s a culture here that hospitals don’t buy ECMO machines. They lease machines and buy only the disposables. In the rest of Latin America, institutions purchase the entire system,” he says. “With a local manufacturer, it will be easier to maintain machines and we won’t need to import parts, so the culture could change.”
* Pesquisa FAPESP magazine
