Novel material can be used to oxidize water molecules and produce hydrogen
07 de março de 2023FAPESP Innovative R&D* – Photoelectrocatalysis is a method of producing energy using semiconductors (materials that transport energy) activated by sunlight or artificial light. The many applications of this method, which has been called artificial photosynthesis, include the production of fuels, electricity and hydrogen, and the purification of water by oxidizing undesirable substances.
Several studies in this area have been conducted at the Center for Development of Functional Materials (CDMF), a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP and hosted by the Federal University of São Carlos (UFSCar) in São Paulo state, Brazil.
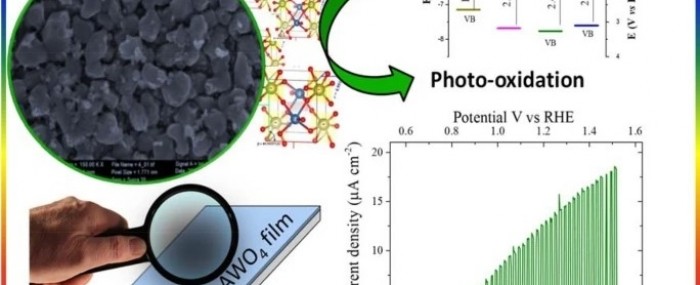
Recent results are reported in an article published in partnership with scientists at Piauí State University (UESPI) in the Journal of Applied Electrochemistry. The article describes an easily implemented method to prepare thin films of transition metal tungstate (AWO4) and highlights the remarkable photoelectrochemical properties of the material.
The films were deposited on a conductive glass substrate coated with fluorine-doped tin oxide (FTO). Analysis by X-ray diffraction showed the presence of crystalline films, and field emission scanning electron microscopy images revealed nanostructured materials.
In addition, AWO4 films were successfully used in a photoelectrochemical cell as photoanodes (cell components that use sunlight to oxidize water, releasing hydrogen and oxygen).
According to the authors, the results show that the films can be used as photoanodes in water splitting reactions and other photoelectrocatalytic applications.
The article “Transition metal tungstates AWO4 (A2+ = Fe, Co, Ni, and Cu) thin films and their photoelectrochemical behavior as photoanode for photocatalytic applications” is at: link.springer.com/article/10.1007/s10800-023-01851-w.
* With information from CDMF, a Research, Innovation and Dissemination Center funded by FAPESP.
