Brazilian startup develops advanced artificial ventilation monitoring system
26 de junho de 2018By Suzel Tunes | FAPESP Research for Innovation – Timpel, a startup based in São Paulo, Brazil, makes a system for monitoring mechanical ventilation that is designed for use in precision medicine. Its name is a Portuguese-language acronym for electrical impedance tomography (EIT), a technique used to monitor intensive-care patients who require artificial respiration.
The system was developed during several years of research. Timpel’s scientists now aim to upgrade the technology via a project supported by FAPESP’s Innovative Research in Small Business Program (PIPE). The goals of the project are development of a new computerized device to support clinical decisions on lung protection, and proof of its effectiveness.
To this end, Timpel has participated in several studies, including an alveolar recruitment trial (ART) led by the São Paulo Heart Hospital (HCor). According to electrical engineer Rafael Holzhacker, Timpel’s president, the studies compare two different strategies used in patients with hypoxemia (low blood oxygen): the conventional treatment (ARDSnet), and the open lung approach (OLA).
He explains that when a patient is bed-bound with a respirator for a long time, the posterior part of the lung begins to close up under the effect of gravity. The alveoli collapse, or open and close during ventilation, causing a dangerous inflammatory response. To avoid these adverse events, the OLA consists of keeping the lung “open” by maintaining sufficient inspiratory pressure to prevent the alveoli from closing (recruitment).
According to Holzhacker, the OLA was not considered secure before EIT existed because medical staff feared causing some kind of injury and making the patient’s condition worse. They preferred to use the standard ventilation strategy known as ARDSnet (acute respiratory distress syndrome network), which prioritizes low-volume ventilation.
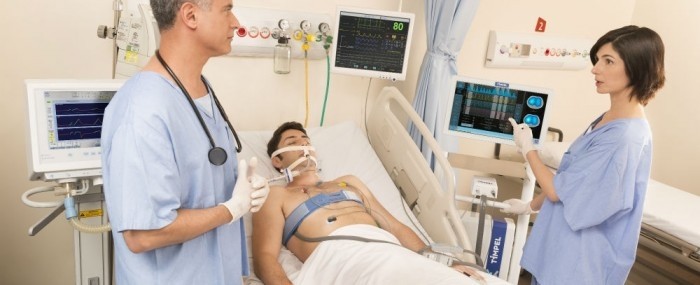
EIT monitoring of mechanical ventilation helps medics choose the safest treatment for the patient in a personalized and assertive manner. “Our equipment tells the doctor immediately if the selected strategy is working and provides timely warning of possible complications. Users are looking for precision ventilation suited to the needs of each patient,” he says.
Lung disorders can also be diagnosed by CT scan, using X-rays to produce high-resolution images. “But this entails the risks associated with radiation, as well as the need to move the patient to the radiology room, increasing the complexity of the treatment and the cost to the health system, whereas with EIT, the patient can remain in bed, and scans can be performed as often as necessary. It doesn’t emit X-rays,” Holzhacker explains.
The measurements made by Timpel’s EIT module are based on resistance to electric current flow (impedance) in different parts of the body. Images are obtained by means of a chest belt with 32 electrodes similar to those used to produce an electrocardiogram. When the machine is switched on, a weak electric current passes through the patient’s chest and highlights the region in which air is circulating as it meets differences in resistance on the way.
An image of the respiratory flow is produced, enabling the physician to control air volume and pressure and hence to choose the optimal ventilation strategy for each patient. As an additional aid to medical staff, Timpel’s researchers are developing clinical decision support software that evaluates the patient’s lung recruitability.
“The doctor performs a simple test by injecting air at a certain pressure for the software to estimate the probability of a positive patient response to the protective ventilation strategy,” Holzhacker says. “This support tool is important because it enables each patient to receive the most suitable treatment at each moment. No one will receive higher pressure if this isn’t beneficial or have to suffer from a collapsed lung subject to inflammation if they’re capable of responding well to protective ventilation.”
Timpel currently has more than 80 modules in service in Brazil and also in the US, Canada, Chile, Peru, Czech Republic, France, Germany, Italy, Spain, Sweden, and Japan. The first units were sold in 2015, after the firm won approval from ANVISA, Brazil’s national health surveillance agency, and from the European Union. In the US, the EIT platform is being marketed as a clinical research tool while awaiting approval from the Food & Drug Administration (FDA).
From lab to business
Timpel’s EIT system is the result of several years of partnership with the University of São Paulo’s Medical and Engineering Schools, as well as the Federal University of the ABC (UFABC). Their first studies on new artificial ventilation techniques date from 2002 and discuss the need for a device to monitor treatment strategies continuously and in an individualized manner.
In 2003, a prototype EIT module was made as part of a Thematic Project funded by FAPESP, using components available in the marketplace. The firm was born in 2004 to develop the machine. Initially, it was incubated at the University of São Paulo’s Center for Innovation, Entrepreneurship & Technology (CIETEC). In 2006, the device was already in experimental use at Hospital das Clínicas, the teaching hospital attached to the University of São Paulo’s Medical School (HCFM-USP), to monitor intensive-care patients.
The firm currently has a staff of 35, about half of whom are dedicated to research and development. Its main office is in São Paulo, and it has a branch in the Netherlands with a sales force and clinical support team to service customers in Europe.
Holzhacker says Timpel will continue to improve the platform with the aim of expanding sales in Brazil and abroad. “We want it to have an ever-growing range of functions and become indispensable in ICUs,” he says.
Company: Timpel
Site: www.timpel.com.br
Contact: info@timpelmedical.com
