Technology reduces need for extracorporeal ventilation in patients with acute respiratory failure by 80%
12 de abril de 2021By Elton Alisson | Agência FAPESP – An electrical impedance tomography device developed by Timpel, a health technology company headquartered in São Paulo, Brazil, helped the critical care team at Massachusetts General Hospital (MGH) in Boston, United States, bring about an 80% reduction in the use of extracorporeal membrane oxygenation therapy (ECMO) for patients with acute respiratory failure.
ECMO is an advanced artificial ventilation technique and can be used as life support in critical cases of COVID-19. The blood exits and returns to the patient’s body via catheters, and is oxygenated in the machine with the aid of a polymeric membrane.
An article on the MGH team’s experience is published in the journal Respiratory Care.
“The hospital has a lung rescue team that has used our device since 2016 and has been obtaining spectacular results,” said Timpel CEO Rafael Holzhacker in a presentation to a webinar on “Scientific entrepreneurship and innovation in response to COVID-19” held by FAPESP with the support of the Global Research Council (GRC) on April 7.
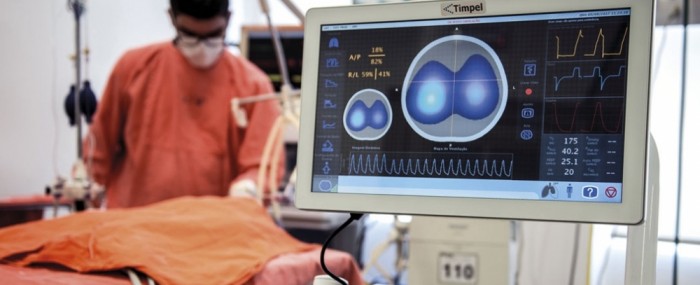
The electrical impedance tomography (EIT) system developed by the firm, with support from the FAPESP Innovative Research in Small Business (PIPE), enables the condition of the patient’s lungs to be monitored non-invasively at the bedside, helping the critical care team optimize mechanical ventilation, reducing complications, preventing lung damage, and keeping the intervention as short as possible.
“Mechanical ventilation is complex and non-intuitive, entailing risks that aren’t visible from the bedside, and patients’ responses are very heterogeneous,” Holzhacker said.
Patients progress slowly while intubated, and the mechanical ventilation strategy adopted in one case may not work in another. “For this reason, it’s very important for the critical care team to have individualized indicators while imaging the patient’s lungs. The goal is to manage mechanical ventilation appropriately so as to reduce the amount of time during which the patient depends on the machine to breathe and mitigate the side-effects of intubation,” he stressed.
Timpel’s EIT system measures electrical impedance (resistance to current flow) in the lungs, which varies considerably as the patient breathes in and out. A low-intensity current similar to that used to produce an electrocardiogram passes through a chest belt with 32 electrodes, highlighting the region in which air is circulating as it meets differences in resistance on the way. Based on the impedance measured on the surface of the chest, the device generates 50 images per second presenting the doctor at the bedside with vital real-time information on the distribution and dynamics of lung insufflation. Onboard software developed during the project supported by PIPE-FAPESP enables the critical care team to decide which ventilation strategy bests protects the patient.
With the aid of Timpel’s EIT machine, the lung rescue team at Massachusetts General Hospital developed individualized mechanical ventilation strategies for 15 patients with severe acute respiratory failure for whom ECMO was indicated.
By means of ventilation maneuvers viewed using the EIT device, the team succeeded in avoiding the need for ECMO in all but two of the 15 patients concerned.
“ECMO is one of the last resorts used in ICUs because it’s expensive and very complex, but the need for this therapy has increased enormously during the COVID-19 pandemic,” Holzhacker said.
In another article published early in 2020 in the journal Critical Care, the MGH team reported that the risk of death for intubated obese patients with acute respiratory failure was reduced by half for up to a year, thanks to individualized treatment made possible by Timpel’s EIT system.
“Our connection with the medical team at MGH, Harvard Medical School’s largest hospital, and with other hospitals not just in the United States but also in Brazil, Italy and Spain, has been vitally important to our response to the demand fueled by the COVID-19 pandemic,” Holzhacker said.
Another contributing factor was the wide array of EIT applications developed before the pandemic, he added, for obese patients (one of the groups at risk of progressing to the severe form of the disease), to assess the effect of prone positioning (ventilation with the patient lying face down), and for children and newborns, among other uses.
Versatile platform
The versatility of a technology used to produce recombinant proteins that are hard to express has enabled researchers at the biotech startup Biolinker to develop COVID-19 diagnostic tests and SARS-CoV-2 detection kits.
The technology is based on cell-free protein synthesis, invented about 60 years ago and used to decode the human genome but still expensive. Biolinker’s researchers have refined it in stages to produce and purify recombinant proteins continuously.
“We’re the second company in the world capable of lyophilizing the cell-free system. That’s important because it enhances protein stability and yield, as well as facilitating transportation,” said Mona das Neves Oliveira, founder of the firm, which is incubated at the Center for Innovation, Entrepreneurship and Technology (CIETEC), jointly run by the University of São Paulo (USP) and the Nuclear and Energy Research Institute (IPEN).
Following the emergence of COVID-19, Biolinker’s researchers realized that the platform they had developed could produce lyophilized SARS-CoV-2 proteins for use in the development of vaccines, drugs, and diagnostic tests. These proteins include the nucleocapsid antigen of the surface-expressed spike protein used by the virus to bind to the ACE2 receptor and infect human cells, and the receptor-binding domain protein (RBD) at the docking tip of the spike.
The nucleocapsid antigen is being used in an ELISA (enzyme-linked immunosorbent assay) test under development by Biolinker in partnership with Ester Sabino, a professor and researcher at the University of São Paulo’s Institute of Tropical Medicine (IMT-USP), via a project supported by PIPE-FAPESP.
The test is designed to detect the presence of immunoglobulin G (IgG) antibodies in blood serum. These are produced in the acute stage of the disease, on average ten days after the onset of symptoms (read more at: agencia.fapesp.br/33500).
The test has been validated by IMT-USP on blood samples from 250 patients treated at Hospital das ClÃnicas, the hospital complex run by the University of São Paulo’s Medical School (HC FM-USP). “We succeeded in obtaining very interesting results. The data shows the test we developed has a sensitivity of 95% to detect IgG antibodies, better than the commercially available tests,” Oliveira said.
The RBD protein is used in a low-cost COVID-19 test the firm developed in partnership with Frank Crespilho, a professor and researcher at the University of São Paulo’s São Carlos Institute of Chemistry (IQSC-USP) (read more at: agencia.fapesp.br/35101/).
“This protein is important to assess the effectiveness of COVID-19 vaccines because it detects neutralizing antibodies, but it’s extremely hard to express. We developed strategies to maintain its stability and improve its response and sensitivity,” Neves said.
Both tests are nearing completion of trials to obtain approval by ANVISA, Brazil’s national health surveillance agency.
More recently, the firm established a partnership with IPEN researchers Ligia Morganti and Carlos Roberto Jorge Soares to produce the entire SARS-CoV-2 spike protein. “It’s a very large protein and hard to express. We are producing it in human cells,” Neves said.
Culture of innovation
Besides Biolinker and Timpel, other firms supported by PIPE-FAPESP have been at the forefront of efforts to develop solutions that contribute to the fight against COVID-19. They include Biologix and Hoobox, both of which are supported by Eretz, a health tech incubator operated by the Albert Einstein Jewish-Brazilian Charitable Society (SBIBAE). SBIBAE also operates the Albert Einstein Jewish-Brazilian Hospital, one of the largest private hospitals in Brazil.
An Internet of Things-based system developed by Biologix to diagnose and monitor sleep apnea in the home has been found effective for remote monitoring of individuals with suspected COVID-19 or mild symptoms of the disease. The system can also be used to recommend transfer to a hospital only if the patient’s clinical signs worsen (read more at: pesquisaparainovacao.fapesp.br/1381).
Hoobox partnered with Radsquare, a spinoff from HIAE, to develop a system that combines artificial intelligence and computer vision for facial recognition to detect people with fever at a distance. The hospital uses the system to monitor visitors as they approach the front desk (read more at: pesquisaparainovacao.fapesp.br/1367).
“The system is called Fevver and was developed in partnership with this hospital,” said Rodrigo Bornhausen Demarch, HIAE’s Chief Innovation Officer, and co-founder and CEO of Sao Paulo-based health tech Zetta Health Analytics.
Eretz is the best-known part of HIAE’s innovation activities, which go much farther and include a unit called Design Lab. “Promotion of our innovation initiatives within the organization starts with Design Lab,” Demarch said.
Management of the innovation process, intellectual property rights (which include technology transfer and licensing), and partnering with companies and universities are the responsibility of HIAE’s Business Technology Center (BTC).
Eretz is the third pillar of the organization’s innovation efforts, Demarch explained. Originally a startup incubator, it has expanded to become an innovation and health entrepreneurship ecosystem currently supporting 76 companies, 70% in digital health, 15% in biotechnology, and the rest developing medical devices.
Incubated startups receive support in marketing, business development, training, protection of intellectual property, funding from angel investors and research agencies, and technological innovation.
“Startups incubated by Eretz have raised more than BRL9 million for the development of solutions to combat coronavirus,” Demarch said, adding that health tech entrepreneurship is now fairly mature in Brazil and that innovations in healthcare must be science-based.
“A good health tech must be grounded in science first and foremost in the vast majority of cases, and this requires an exploratory process to identify an unresolved clinical problem,” he stressed.
A complete recording of the webinar (in Portuguese) can be watched at: covid19.fapesp.br/488.
